Science underpins the quality of all our next generation products.
That’s why it’s important that both ourselves and the wider industry evaluate the scientific methods we use to assess our products and find ways to improve them.
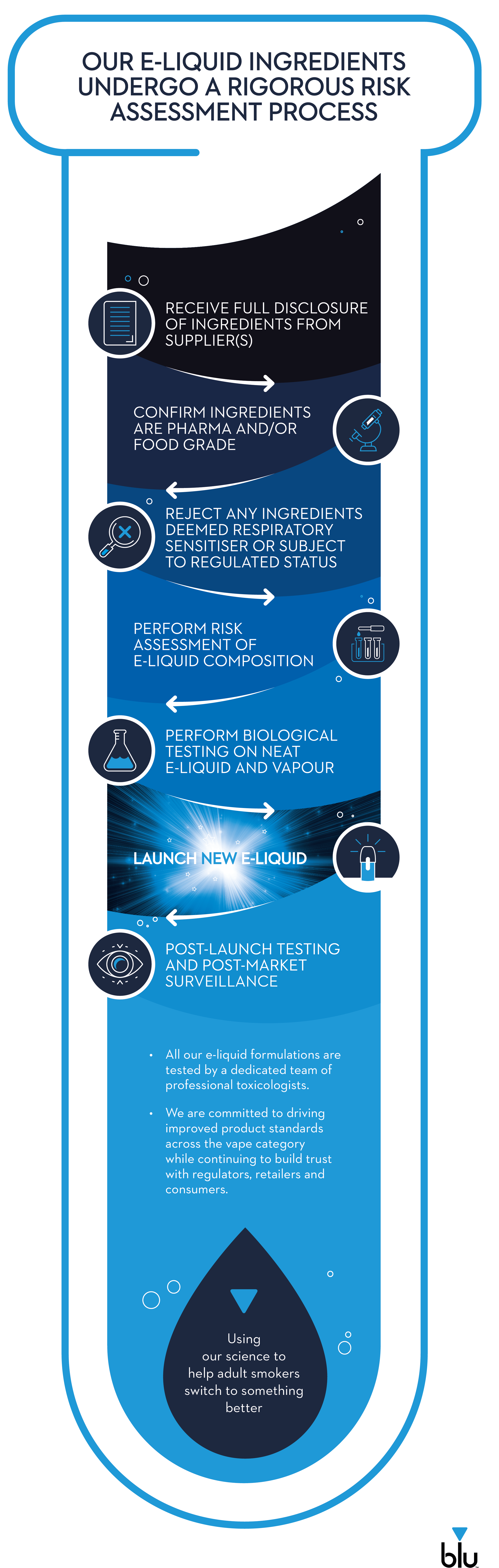
Imperial Brands is committed to high product quality standards across all NGPs, including in vape e-liquids, which already undergo rigorous testing by professional toxicologists through our risk assessment process, which is detailed below.
 However, in innovative new research, Imperial Brands scientists have shown how Genomic Allergen Rapid Detection (or GARD) in-vitro assays could be used to differentiate and classify vape e-liquids, as well as detect allergens potentially present in ingredients.
However, in innovative new research, Imperial Brands scientists have shown how Genomic Allergen Rapid Detection (or GARD) in-vitro assays could be used to differentiate and classify vape e-liquids, as well as detect allergens potentially present in ingredients.
Demonstrating our continued commitment to high-quality products, these promising results show that such assays could, in future, be applied to e-liquid testing to raise industry standards.
GARD assay testing represents another important step in our commitment to cutting-edge e-vapour research, and forms part of our ambition to build a next-generation scientific assessment framework partly based on the US National Research Council’s blueprint for Toxicity Testing in the 21st Century, or TT21C.
The availability of a broad range of e-liquids plays a crucial role in both attracting adult smokers to vaping and retaining them within the category. This, in turn, contributes to declining smoking rates and potential tobacco harm reduction. However, it’s important that e-liquid flavourings and their ingredients are guided by toxicological principles.
These assays were originally developed to assess the sensitisation potential of compounds in the chemical manufacturing industry, but this pioneering research is the first vaping-related application.
For the study, scientists used the GARD testing approach to compare the respiratory and skin sensitising potential of five e-liquids, with initial results indicating the assays were indeed successfully able to differentiate and classify those products assessed.
Lukasz Czekala, In-Vitro Research Toxicologist and study author, comments:
“While further studies need to be conducted to assess how GARD assays could be used for the rapid screening and toxicological assessment of e-liquids in support of future product development and commercialisation, our initial success represents an exciting first step on the path to a potential entirely new way of assessing and classifying future e-liquids.”
“TT21C advocates the use of in-vitro testing, preferably using human cells, as a replacement for traditional in-vivo animal testing and this is in keeping with Imperial’s established position of not testing our products on animals.
“By uplifting our scientific capabilities through the likes of TT21C we are continuing to deliver quality products to our consumers while raising standards across the wider vaping category.”
You are free to share this content with credit to Imperial Brands under a Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license.
