Imperial Brands continues to investigate the application of innovative in-vitro methodologies to assess the harm reduction potential of our Next Generation Product (NGP) portfolio, relative to combustible tobacco.
In this latest study, published in the journal Current Research in Toxicology, a range of NGP aerosols – including our myblu vape product, as well as cigarette smoke[1] – were captured in phosphate buffered saline.
They were then exposed to a panel of 12 human relevant primary cell-based model systems using Biologically Multiplexed Activity Profiling (BioMAP),[2] an assay often used to screen for unexpected side-effects during pharmaceutical drug development.
Following exposure to the cell systems, the biological activity for a wide range of biomarkers were further analysed[3]. The objective was to determine whether a range of toxicity signatures associated with a group of specific biomarkers relating to smoking-related disease endpoints were present in any of the products tested, with the results as follows:
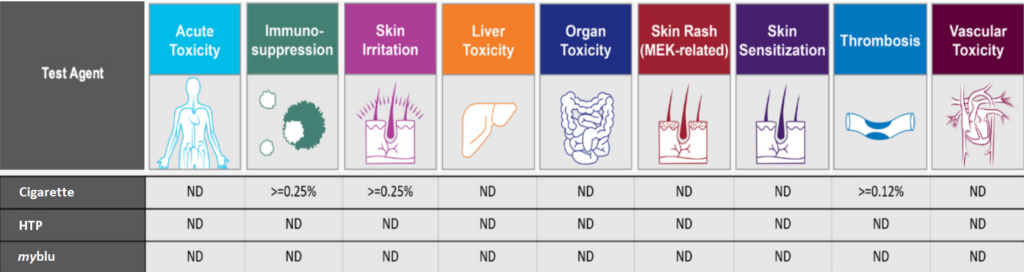
Figure 1. Toxicity Signature Analysis for the Diversity PLUS panel. Evaluation of the presence of Toxicity Signatures within the BioMAP profile of the tested article and concentrations detected. Toxicity Signatures are made up of 2–5 biomarker activities that have been correlated to an increased risk of certain toxicity effects in-vivo. Concentrations are listed if the signature for the toxicity was detected. Not detected (ND) indicates the signature was not detected at any of the concentrations tested.
Liam Simms, Principal Toxicologist and study author, commented: “Imperial Brands is committed to making a meaningful contribution to tobacco harm reduction, and these results, from the first published application of this assay for NGP research, are encouraging for several reasons.
“Firstly, they add to the weight of existing in-vitro evidence supporting the reduced toxicity potential of NGPs compared to combustible cigarette smoke.
“Secondly, as part of our Alternatives to Animal Testing approach we’re committed to exploring new models to further substantiate the harm reduction potential of our NGP portfolio, based on TT21C methodologies.
“The growing scientific evidence base indicates a range of human relevant, cell-based assays like BioMAP – validated by the appropriate clinical studies – have the potential to render traditional in-vivo animal testing redundant.
“This is another positive step forward – not only in terms of the validation of our NGP testing, but also potentially in terms of accelerating the transition away from animal testing in laboratories”.
[1] From a 3R4F reference cigarette
[3] Via Eurofins Discovery Toxicity Signature Analysis
[4] Arnson et al., (2010); Sorenson et al., (2010); Tapson (2005)
You are free to share this content with credit to Imperial Brands under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license.
