//Tobacco-free oral nicotine pouch clinical study update
Posted 09/05/2022 9:17am
Imperial Brands has conducted a clinical assessment of its tobacco-free nicotine pouch products. Clinical Research Senior Manager Simon McDermott presents a summary of the data
Among the Next Generation Products (NGPs) with the potential to make a meaningful contribution to the public health concept of tobacco harm reduction (THR) are tobacco-free oral nicotine pouches.
Free from tobacco combustion and the generation of high levels of harmful chemicals and smoke toxicants, high quality nicotine pouches offer those adult smokers who are unwilling or uninterested to stop using combustible cigarettes a potentially harm-reduced alternative. Its appearance and oral method of use is similar to traditional tobacco-containing Scandinavian snus, itself a harm-reduced product supported by favourable epidemiological data in Sweden (see our snus infographic for more information).
One vital difference makes nicotine pouches distinct: they are completely free of tobacco leaf, instead obtaining their nicotine content via high purity pharmaceutical grade nicotine within a matrix of dry powder or a plant fibre-based substrate.
Without tobacco and combustion, their THR potential relative to combustible cigarettes and snus may be even greater.
Building on an encouraging 2021 summary of key scientific findings, our scientists recently finished conducting a further clinical study with more nicotine pouch products from our zoneX/SKRUF Superwhite portfolio.
This ethics-approved clinical trial enrolled 27 adult subjects – all existing tobacco-free oral nicotine pouch or tobacco-snus users – into the study clinic for 4 days in Sweden. They were each randomly assigned to receive a different product per day from the following nicotine products: 14.4mg of nicotine per pouch, 16mg per pouch and 20mg per pouch (the voluntarily maximum nicotine strength limit in the EU, agreed by responsible manufacturers). A tobacco-snus comparator product was also supplied, containing 16.6mg of nicotine per pouch.
Subjects’ blood samples were collected before each product usage session began. These sessions lasted 20 minutes, as recommended in our product usage guidance, after which subjects’ blood samples were again collected and measured. Subjects were also asked to complete a series of questionnaires to assess satisfaction and product liking levels, and to help us understand if these products help to reduce urges to use nicotine. The study aims involved:
- Assessing the products’ short-term safety and tolerability in a controlled clinical setting.
- Better understanding their pharmacokinetic profiles, including the blood nicotine delivery of nicotine pouches compared to the tobacco-snus product.
- Assessing subjects’ subjective feelings towards the products (e.g. levels of urge to use, satisfaction, liking, relief etc.)
The results were as follows:
- All products (with a strength up to 20mg per pouch) had a favourable short term safety and tolerability profile under the conditions of the clinical study.
Under the conditions of study procedure (20 mins per session, up to 20mg per pouch), no significant negative impacts on subjects’ blood pressure, pulse, heart measurements or general wellbeing were observed. There were a small number of adverse effects (AEs) recorded as possibly related to the product, though these were all mild and commonly reported for nicotine-containing products.
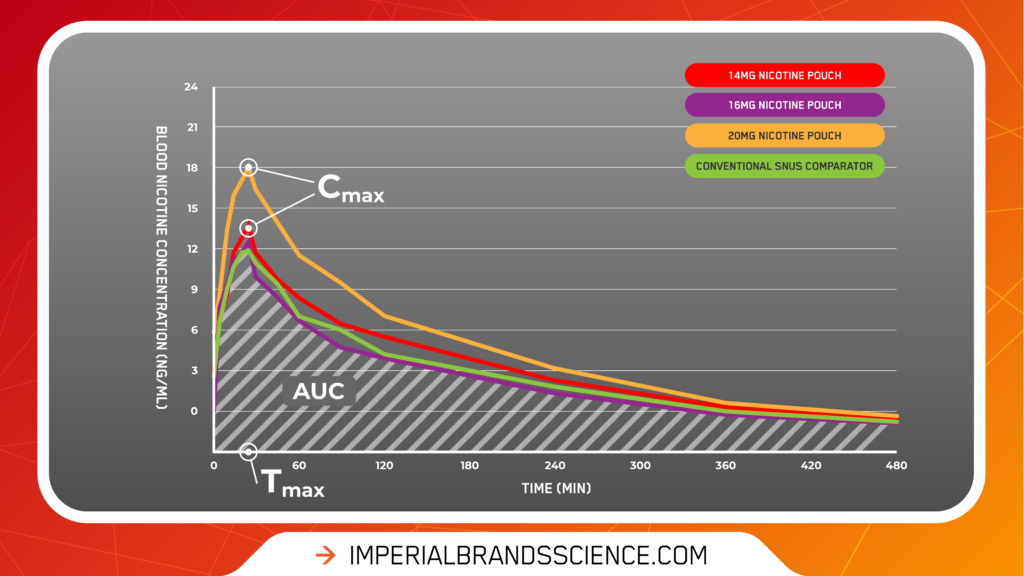
- All products deliver nicotine efficiently to the blood via the oral mucosa with no secondary nicotine peaks during the observation period, which would be indicative of nicotine absorption via swallowing.
- Cmax (the maximum concentration of nicotine in the blood stream) data indicate that nicotine pouches deliver nicotine efficiently to the bloodstream via the oral mucosa. There was a lack of any secondary nicotine peak, which would likely indicate some gastro-intestinal nicotine absorption had taken place through subjects swallowing. In line with our previous findings, this indicates nicotine is delivered to the bloodstream via the oral mucosa (buccal absorption).
- Tmax (the time taken to reach the maximum nicotine concentration) was, on average, achieved at 25 min – which was closely aligned with most consumers’ usage time per pouch.
- The Area under Curve (AUC, which denotes a subject’s total exposure to nicotine per pouch) was – as expected – found to be broadly proportional to the nicotine content of the pouch (i.e. as nicotine strength increases, so do blood nicotine concentration levels).
The graph below helps bring the data to life.
- All products offer a satisfying alternative to traditional tobacco containing products and reduce users’ urge to use nicotine
After initial use of each product, the subjects urge to use nicotine reduced significantly when compared to baseline levels, with the maximum reduction in urges (TE-max) occurring at around 20-25 minutes – illustrated by the graph below.
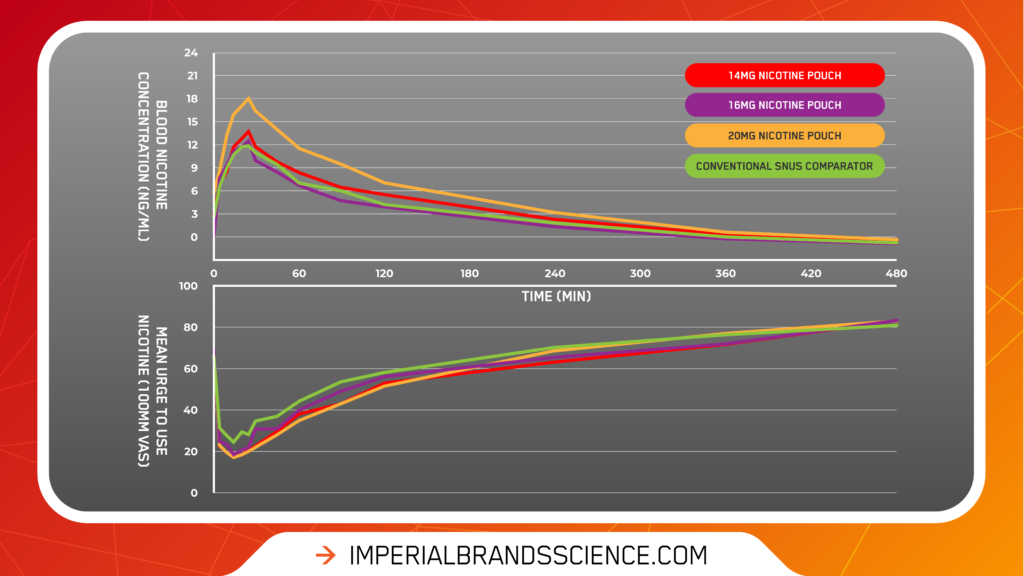
Subject questionnaire results also included encouraging scores relating to product satisfaction, reward and relief across all pouch strengths.
Overall, these latest encouraging results positively contribute to the existing scientific evidence base substantiating the favourable short-term safety and tolerability, efficient blood nicotine delivery and user satisfaction scores of zoneX/Skruf Superwhite tobacco-free oral nicotine pouches with a pouch strength of up to 20mg.
Please look out for further research soon.
You are free to share this content with credit to Imperial Brands under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license.

